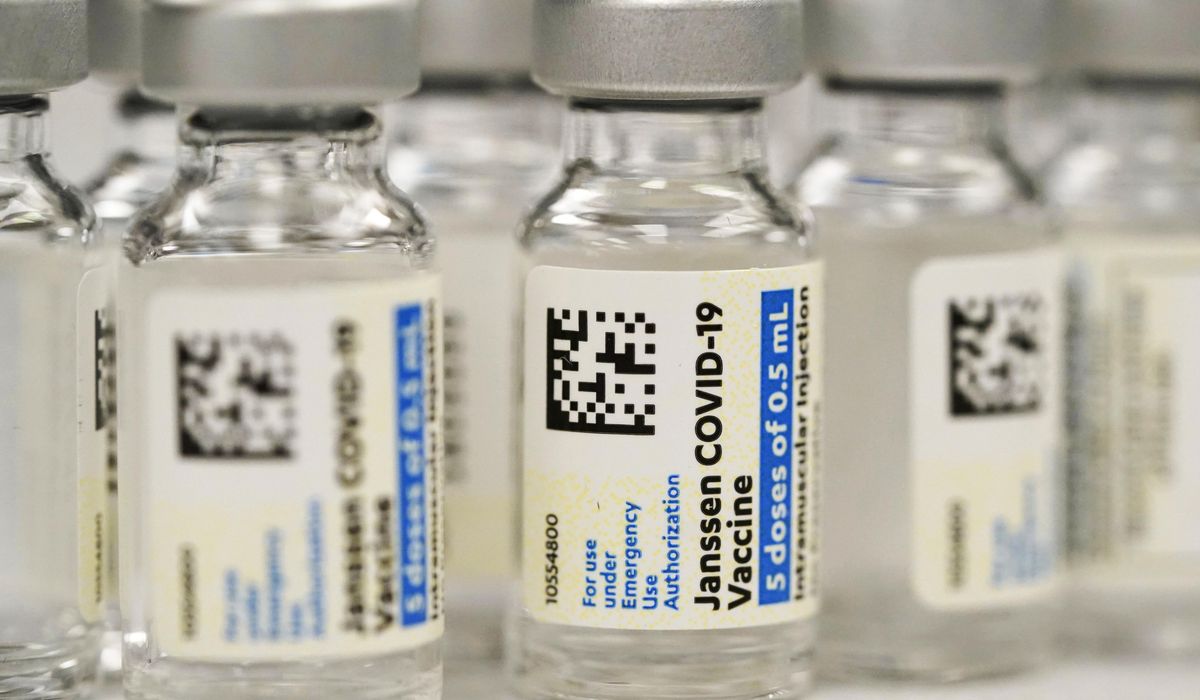
The Meals and Drug Administration mentioned Thursday it’s limiting the usage of the COVID-19 vaccine from Johnson & Johnson to adults who can’t entry one other model or would possibly face a medical challenge in the event that they take one other sort.
Regulators cited the danger of a uncommon however extreme blood-clotting situation that’s been linked to the J&J vaccine, with 60 recognized circumstances and 9 deaths.
It’s one other setback for the J&J vaccine, which confirmed promise as an environment friendly one-shot possibility earlier within the pandemic, however confronted manufacturing hiccups and questions on security and efficacy versus the messenger RNA vaccines from Pfizer-BioNTech and Moderna which have turn out to be the dominant choices.
“We acknowledge that the Janssen COVID-19 Vaccine nonetheless has a task within the present pandemic response in america and throughout the worldwide neighborhood. Our motion displays our up to date evaluation of the danger of [thrombosis with thrombocytopenia syndrome] following administration of this vaccine and limits the usage of the vaccine to sure people,” mentioned Peter Marks, director of the FDA’s Heart for Biologics Analysis and Analysis.
Fewer than 20 million of the J&J photographs have been administered within the U.S., and it accounts for lower than 4% of major vaccinations within the U.S. It additionally accounts for a small fraction of booster photographs.
The vaccine has been a part of the trinity of vaccines used within the U.S. since early 2021, nevertheless it was paused for a time in early 2021 as regulators investigated the uncommon clotting challenge, or TTS.
It additionally confronted manufacturing issues attributable to a mix-up at a Baltimore plant, and regulators beforehand mentioned the mRNA vaccines had been preferable to the J&J vaccine due to lingering issues about TTS.
The FDA on Thursday mentioned it took even stricter motion as a result of it feared charges of TTS weren’t trailing off.
“The FDA has decided that the recognized and potential advantages of the vaccine for the prevention of COVID-19 outweigh the recognized and potential dangers for people 18 years of age and older for whom different licensed or authorised COVID-19 vaccines usually are not accessible or clinically acceptable, and for people 18 years of age and older who elect to obtain the Janssen COVID-19 Vaccine as a result of they’d in any other case not obtain a COVID-19 vaccine,” the company mentioned.
The Washington Instances reached out to J&J for touch upon the FDA’s determination.
For extra data, go to The Washington Instances COVID-19 useful resource web page.







_updates.jpg)